Experimental and theoretical evidence for multiple FeIV reactive intermediates in TAML-activator catalysis: Rationalizing a counterintuitive reactivity order
Abstract
Reacting rationally
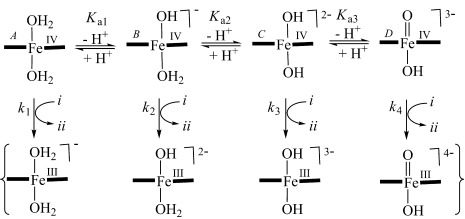
During the 1e oxidation of ferrocyanide by the catalytic TAML activator and H2O2, four FeIV tetra-amido macrocyclic ligand (TAML) intermediates were detected that are involved in a fast acid–base equilibrium. The counterintuitive reactivity pattern is explained by the overall free-energy change during the reduction of FeIV to FeIII TAML complexes, with competing contributions from electronic and solvation energy changes.
Type
Publication
Chemistry A European Journal