Metatranscriptomic investigation of adaptation in NO and N2O production from a lab-scale nitrification process upon repeated exposure to anoxic–aerobic cycling
Abstract
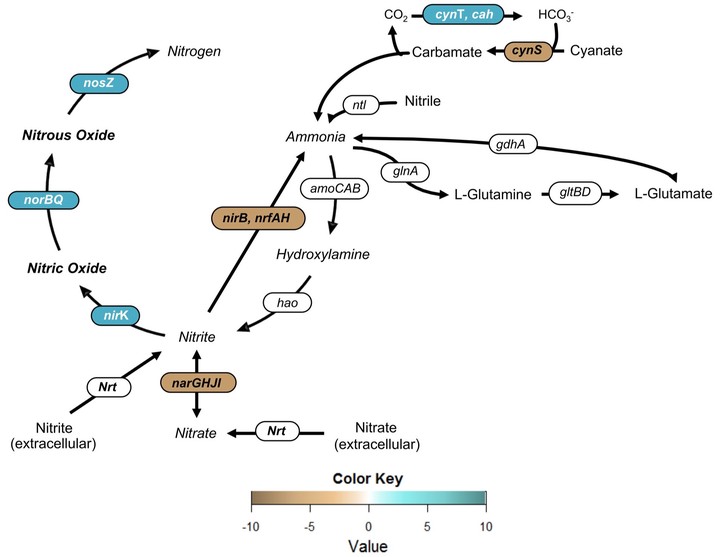
The molecular mechanisms of microbial adaptation to repeated anoxic–aerobic cycling were investigated by integrating whole community gene expression (metatranscriptomics) and physiological responses, including the production of nitric (NO) and nitrous (N2O) oxides. Anoxic–aerobic cycling was imposed for 17 days in a lab-scale full-nitrification mixed culture system. Prior to cycling, NO and N2O levels were sustained at 0.097 ± 0.006 and 0.054 ± 0.019 ppmv, respectively. Once the anoxic–aerobic cycling was initiated, peak emissions were highest on the first day (9.8 and 1.3 ppmv, respectively). By the end of day 17, NO production returned to pre-cycling levels (a peak of 0.12 ± 0.007 ppmv), while N2O production reached a new baseline (a peak of 0.32 ± 0.05 ppmv), one order of magnitude higher than steady-state conditions. Concurrently, post-cycling transcription of norBQ and nosZ returned to pre-cycling levels after an initial 5.7- and 9.5-fold increase, while nirK remained significantly expressed (1.6-fold) for the duration of and after cycling conditions. The imbalance in nirK and nosZ mRNA abundance coupled with continuous conversion of NO to N2O might explain the elevated post-cycling baseline for N2O. Metatranscriptomic investigation notably indicated possible NO production by NOB under anoxic–aerobic cycling through a significant increase in nirK expression. Opposing effects on AOB (down-regulation) and NOB (up-regulation) CO2 fixation were observed, suggesting that nitrifying bacteria are differently impacted by anoxic–aerobic cycling. Genes encoding the terminal oxidase of the electron transport chain (ccoNP, coxBC) were the most significantly transcribed, highlighting a hitherto unexplored pathway to manage high electron fluxes resulting from increased ammonia oxidation rates, and leading to overall, increased NO and N2O production. In sum, this study identified underlying metabolic processes and mechanisms contributing to NO and N2O production through a systems-level interrogation, which revealed the differential ability of specific microbial groups to adapt to sustained operational conditions in engineered biological nitrogen removal processes.
*Contributed equally